What thermal efficiency was James Watt's 1765 steam engine able to achieve?
A. 1%
B. 4%
C. 10%
D. 40%
Answer: B
You might also like to view...
An ideal gas, initially at 30°C and 100 kPa, undergoes the following cyclic processes in a closed system:
What will be an ideal response?
(a) In mechanically reversible processes, it is first compressed adiabatically to 500 kPa, then cooled at a constant pressure of 500 kPa to 30°C, and finally expanded isothermally to its original state.
(b) The cycle traverses exactly the same changes of state, but each step is irreversible with an efficiency of 80% compared with the corresponding mechanically reversible process. Note: The initial step can no longer be adiabatic.
Calculate Q, W, ?U, and ?H for each step of the process and for the cycle. Take 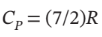 and
and 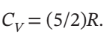
What is the major objection to using an atomizing humidifier with hard water?
What will be an ideal response?
The hot gas line from the heat pump's compressor can be used to ____.
A. cool down the condenser B. heat domestic water C. keep the well water from freezing D. both a and c
Answer the following statement(s) true (T) or false (F)
Rise is the vertical distance of a step, the height of each step.