A particular fully charged battery can deliver 2.7 x106 coulombs of charge. a. What is the capacity of the battery in ampere-hours? b. How many electrons can be delivered?
What will be an ideal response?
Known quantities:
qBattery = 2.7 • 106 C.
Find:
a) The current capacity of the battery in ampere-hours
b) The number of electrons that can be delivered.
Analysis:
a) There are 3600 seconds in one hour. Amperage is defined as 1 Coulomb per second and is directly proportional to ampere-hours.
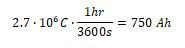
b) The charge of a single electron is -1.602•10-19 C. The negative sign indicates that the particle delivered will be an electron. Simple division gives the solution:
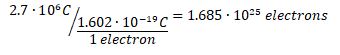
You might also like to view...
Is part number 3 welded all the way around?
Refer to the girder brace in Drawing Number W11784S R1.
Birds used for ____________________ operations have been developed to tolerate confinement and efficiently produce eggs
Fill in the blank(s) with correct word
Demonstrate the phenotypic and genotypic outcome when a black bull that is heterozygous for coat color is mated to a red cow.
What will be an ideal response?
Which memory is nonvolatile yet can be altered and reprogrammed?
A) EPROM B) dynamic RAM C) ROM D) RAM