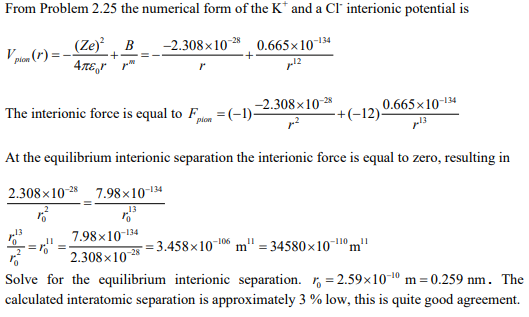
Test the K+ and a Cl– interionic potential that you developed for Problem 2.25 by determining the equilibrium interionic separation, and comparing this value to the experimental value of 0.266 nm.
What will be an ideal response?
You might also like to view...
You have a choice between two lenses of focal lengths fa and fb = 2 fa to use as objective lens in building a compound microscope. If the magnification you obtain using lens A is Ma, what will be the magnification when using lens B?
A) Mb = 2 Ma B) Mb = 4 Ma C) Mb = 8 Ma D) Mb = Ma /4 E) Mb = Ma /2
What element is behaves as the oxidizing agent in the following equation and what element behaves as the reducing agent?
Sn2+ 2 Ag ? Sn + 2 Ag? A) The tin ion, Sn2, is the oxidizing agent while silver, Ag, is the reducing agent. B) The tin ion, Sn2+, is the reducing agent while silver, Ag, is the oxidizing agent. C) The tin, Sn, is the reducing agent while silver ion, Ag?, is the oxidizing agent. D) The tin, Sn, is the oxidizing agent while silver ion,Ag?, is the reducing agent.
Briefly describe the Galilean moons
What will be an ideal response?
The lifetime of a ?+ meson normally is 18 nanoseconds. In a large particle accelerator, scientists can create ?+ mesons moving at speeds very close to the speed of light
What do the scientists observe when they measure the lifetime of a ?+ meson that is created at very high speed? A) The ?+ meson lasts 18 nanoseconds. B) The ?+ meson lasts much more than 18 nanoseconds. C) The ?+ meson lasts much less than 18 nanoseconds. D) The ?+ meson no longer decays and remains as a stable particle.