Compute the change in volume and work done when one kilogram of the
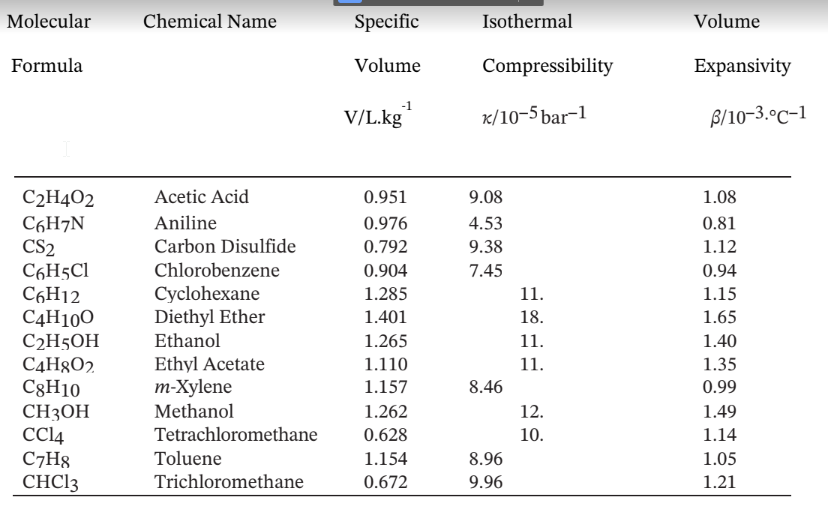
The specific volume, isothermal compressibility, and volume expansivity of several liquids at 20°C and 1 bar25, where ? and ? may be assumed constant.
What will be an ideal response?
Volumetric Properties of Liquids at 20°C
First ethanol will be chosen as the substance to be used. In the problem the pressure is held constant, then the volume expansively equation is used to determine the change in volume.
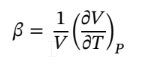
Solving for dv and integrating gives:
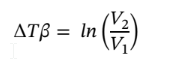
Solving for 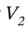 gives:
gives:
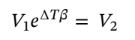
Plugging in the specific volume and volume expansivity leads 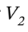 = 1.283 L for 1 kilogram of ethanol.
= 1.283 L for 1 kilogram of ethanol.
To determine the work we know that:
WW = ?P?V = ?1 bar ? (1.283 L ? 1.265 L) = -0.018 bar L = -1.8 joules
You might also like to view...
Which of these is also known as electrical potential?
A) Volts B) Ohms C) Amps D) None of these
Developing sketching ability is not very helpful in the creative efforts of problem solving and design.
Answer the following statement true (T) or false (F)
The peak gain on the closed loop Bode response for a second order lag system should be between 8 and 10 db
Indicate whether the statement is true or false
Refrigerant entering the evaporator is approximately ____________________% liquid.
Fill in the blank(s) with the appropriate word(s).