The Bohr theory of the atom made which of the following BOLD assumptions?
A. The stable orbits are maximally occupied with electrons
B. All atoms contain "special" electrons that do not radiate their energy
C. Electrons in certain orbits do not radiate electromagnetic waves despite being centripetally accelerated
D. The electrons in some orbits are paired so as to become stable
Answer: C
You might also like to view...
Which event in the history of the universe happened last?
A) Stable helium nuclei formed. B) The electroweak force separated into electromagnetic and weak nuclear forces. C) The GUT force separated into the electroweak force and strong force. D) Neutral atoms formed.
An electron gun sends electrons through a region with an electric field of 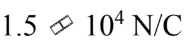 for a distance of 2.5 cm. If the electrons start from rest, how fast are they moving after traversing the gun?
for a distance of 2.5 cm. If the electrons start from rest, how fast are they moving after traversing the gun?
a. 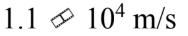
b. 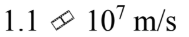
c. 
d. 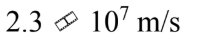
e. 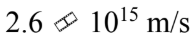
A nonuniform linear charge distribution given by l(x) = bx, where b is a constant, is distributed along the x axis from x = 0 to x = +L. If b = 40 nC/m2 and L = 0.20 m, what is the electric potential (relative to a potential of zero at infinity) at the point y = 2L on the y axis?
a. 19 V b. 17 V c. 21 V d. 23 V e. 14 V
Frank says that quantum mechanics does not apply to baseballs because they do not jump from quantum state to quantum state when being thrown. Francine agrees with him. She says that there is no uncertainty in a baseball's position or momentum. Are they correct, or not, and why?
a. They are correct because the first excited state of a baseball is at a higher energy that any baseball ever receives. Therefore we cannot determine whether or not there is uncertainty in its position or momentum. b. They are correct because the first excited state of a baseball is at a higher energy that any baseball ever receives. Therefore its position and momentum are completely uncertain until it is caught. c. They are wrong because the baseball goes through so many quantum states in being thrown that we cannot observe the transitions. The uncertainties in its position and momentum are too small to observe. d. They are wrong because the baseball goes through so many quantum states in being thrown that we cannot observe the transitions. Because of the number of transitions its position and momentum are completely uncertain until it is caught. e. Quantum mechanics states that they are correct as long as they do not make any observations, but wrong as soon as they begin to make observations.