Latent Heats: A substance has a melting point of 20°C and a heat of fusion of 3.6 × 104 J/kg. The boiling point is 150°C and the heat of vaporization is 7.2 × 104 J/kg at a pressure of one atmosphere. The specific heats for the solid, liquid, and gaseous phases are 600 J/kg ? K (solid), 1000 J/kg ? K (liquid), and 400 J/kg ? K (gaseous). How much heat is given up by 2.80 kg of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
A. 400 kJ
B. 200 kJ
C. 300 kJ
D. 440 kJ
E. 640 kJ
Answer: A
You might also like to view...
A car traveling at 4.0 m/s has a constant acceleration of 2.0 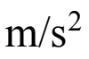 After 3.0 seconds, the distance traveled during the acceleration is
After 3.0 seconds, the distance traveled during the acceleration is
A. 21 m. B. 17 m. C. 10 m. D. 13 m. E. 9 m.
A solid conducting sphere of 10 cm radius has a net charge of 40 nC. If the potential at infinity is taken as zero, what is the potential at the center of the sphere?
a. 360 mV c. >3.6 × 10^4 V b. 3.6 × 10^3 V d. 36 mV
The discovery of Charon was significant because it allowed astronomers to determine the ________ and ________ of Pluto.
A. radius; speed B. age; mass C. speed; distance D. distance; age E. mass; radius
For Galileo, the observation of the phases of ________ proved that Ptolemy's geocentric model with epicycles was wrong
Fill in the blank(s) with correct word