Solve the problem.The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely as the volume of the gas. If the pressure is 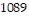 kiloPascals (kPa) when the number of moles is 5, the temperature is
kiloPascals (kPa) when the number of moles is 5, the temperature is 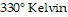 , and the volume is
, and the volume is  , find the pressure when the number of moles is 8, the temperature is
, find the pressure when the number of moles is 8, the temperature is  , and the
, and the
volume is 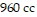 .
.
A. 1782 kPa
B. 1650 kPa
C. 891 kPa
D. 957 kPa
Answer: C
You might also like to view...
Find any relative maximum or minimum points of the given function.y = x4 - 2x2 - 9
A. Maxima at (1, -10), (-1, 8); minimum at (0, -9) B. Minimum at (0, -9), maximum at (1, -10) C. Minima at (1, -10), (-1, -10); maximum at (0, -9) D. Minimum at (-1, -10), maximum at (1, -10)
Provide an appropriate response.The table shows the number of cans of soda sold at a campus stand on five days with different high temperatures for the day. Write each paired data as an order pair of the form 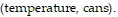 Then create a scatter diagram of the ordered pairs. Do the paired data show a trend. If so, what is the trend?
Then create a scatter diagram of the ordered pairs. Do the paired data show a trend. If so, what is the trend?
What will be an ideal response?
Match the system of equations with one of the graphs.36x2 + 4y2 = 144x + y = 5
A. 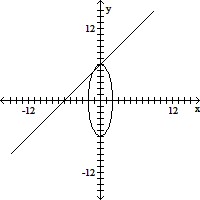
B. 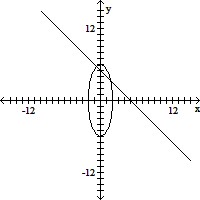
C. 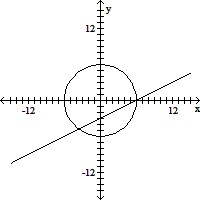
D. 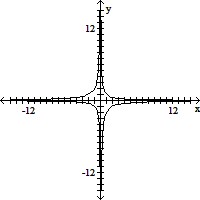
Solve the problem.A student must choose 1 of 5 science electives, 1 of 6 social studies electives, and 1 of 9 language electives. How many possible course selections are there?
A. 270 course selections B. 30 course selections C. 20 course selections D. 540 course selections