One mole of an ideal gas, initially at 30°C and 1 bar, undergoes the following mechanically reversible changes. It is compressed isothermally to a point such that when it is heated at constant volume to 120°C its final pressure is 12 bar. Calculate Q, W, ?U, and ?H for the process.
What will be an ideal response?
For the initial state of 30 °C and 1 bar, the molar volume is
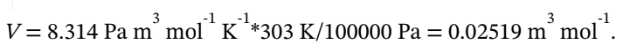
If the final state is 120 °C and 12 bar, then the molar volume at the final state is
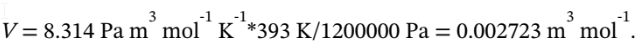
This is also the molar volume at the intermediate state, since the gas goes from the intermediate state to the final state at constant volume. The temperature at the intermediate state is 30 °C, since the gas goes from the initial state to the intermediate state isothermally. So, the pressure at the intermediate state is
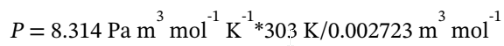 = 925134 Pa = 9.251 bar.
= 925134 Pa = 9.251 bar.
For the isothermal compression, ?U = ?H = 0 and Q = 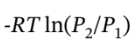 = -R*303 K*ln(9.251/1) = -5605 J/mol, and W = -Q = 5605 J/mol.
= -R*303 K*ln(9.251/1) = -5605 J/mol, and W = -Q = 5605 J/mol.
For the isochoric heating, ?U=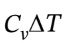 = 2.5 R*(393 K – 303 K) = 1871 J/mol, and ?H =
= 2.5 R*(393 K – 303 K) = 1871 J/mol, and ?H =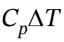 = 3.5 R*(393 K – 303 K) = 2619 J/mol. At constant volume, W = 0, so Q = ?U = 1871 J/mol.
= 3.5 R*(393 K – 303 K) = 2619 J/mol. At constant volume, W = 0, so Q = ?U = 1871 J/mol.
For the overall 2-step process, we then have ?U = 1871 J/mol, ?H = 2619 J/mol,
Q = 1871 J/mol 5605 kJ/mol = -3734 J/mol, and W = 5605 J/mol.
You might also like to view...
How do conventional buildings and green buildings differ?
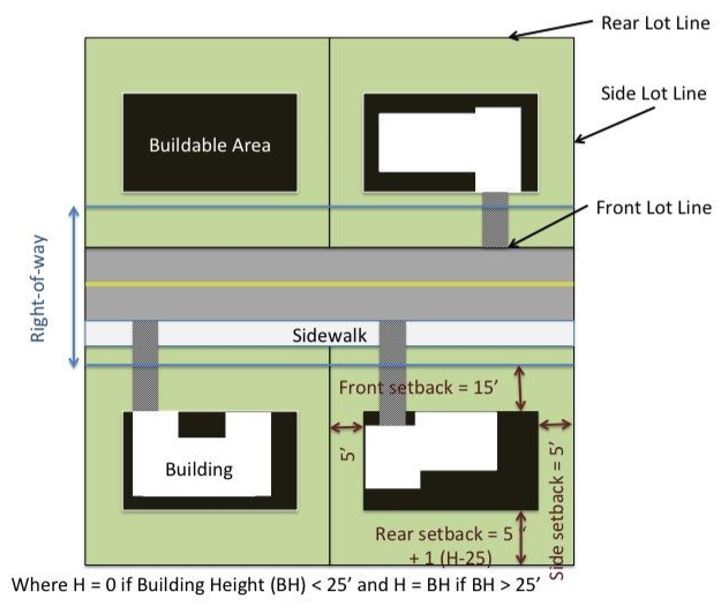
What will be an ideal response?
Assuming ideal gas behavior, determine the mass of each gas listed below, if the gas occupies 0.5 ft3, at 100 psi and 540 R:
(a) hydrogen, (b) methane, (c) nitrogen, (d) argon (e) carbon dioxide Given: V = 0.5 ft3; P = 100 psi; T = 540 R What will be an ideal response?
List four of the recommended components of the abstract
What will be an ideal response?
Which of these components are NOT used to control an electric cooling fan?
A) Engine control module B) Relay C) A/C pressure sensor D) Driver fan switch