An adiabatic free expansion of a gas in a thermally isolated container is not reversible because
a. work must be done on the gas to return it to its original volume.
b. heat must be exchanged with the surroundings to return the gas to its original temperature.
c. its internal energy has a greater value after the expanded gas is returned to its original volume and temperature.
d. of all of the above.
e. of (a) and (b) above only.
a
You might also like to view...
A camera lens is initially set at f/8 for a shutter speed of 1/250 s. If the amount of lighting on the subject is unchanged, and the lens is set at f/4, what is the proper shutter speed at this setting?
a. 1/1000 s b. 1/500 s c. 1/250 s d. 1/2 s e. 1/8 s
A real (non-Carnot) heat engine, operating between heat reservoirs at temperatures of 450 K and 270 K, performs 3.3 kJ of net work, and rejects 8.2 kJ of heat in a single cycle
(a) What is the thermal efficiency of this heat engine? (b) What is the maximum efficiency it could possibly have?
Thermodynamic Devices: If the efficiency of a reversible engine is 28%, what is its COP (performance coefficient) operated (a) as a refrigerator, and (b) as a heat pump?
Fill in the blank(s) with the appropriate word(s).
Series/Parallel Circuits: What is the potential drop from point A to point B for the circuit shown in the figure? The battery is ideal, and all the numbers are accurate to two significant figures.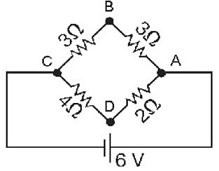
A. 0.35 V B. 2.0 V C. 2.5 V D. 3.0 V