Wainscoting can be any material including wallpaper, sheetrock of varied textures, wood paneling, tile, stone, or masonry used to divide walls into two visual sections.
Answer the following statement true (T) or false (F)
True
You might also like to view...
How many moles of A will be in the reactor at equilibrium?
The gas phase reaction A ? B + C has ?HR0 = 15 kJ/mol and ?GR0 = 10 kJ/mol at 298.15 K. Ten moles of A are placed in a reactor and the system is allowed to come to equilibrium at P = 0.2 MPa and T = 473.15 K. The ideal gas model applies. A. 0 mol B. 2.77 mol C. 7.23 mol D. 8.05 mol E. Insufficient information to solve.
This problem concerns a compound that has the following physical properties:
Critical Temperature = 1000 K Critical Pressure = 100 bar Vapor pressure at 450 K = 4 bar Molar Volume of saturated liquid at 450 K = 300 cm3/mol Enthalpy of vaporization at 450 K = 15 kJ/mol In the VAPOR and GASEOUS states only, it is described by the equation of state: Z = 1 + BP + CP2 Where B = -0.02 bar-1 and C = 0.0005 bar-2 A) Derive an algebraic (i.e., no differentials or integrals) expression for the fugacity of the compound in the gas/vapor state as a function of temperature, pressure, and known constants. B) Use the Clausius-Clapeyron equation to calculate the vapor pressure of this compound at 500 K. C) List the assumptions made in the derivation of the Clausius-Clapeyron equation and comment on how good or bad they are for the example in part B. D) Give your best estimate of the fugacity of this compound in the LIQUID phase at T=500 K and P=50 bar. You may assume your answer to part B is a correct vapor pressure, even if in part C you expressed reservations about its accuracy.
The cut-off ratio is nearest:
An engine operates on a diesel cycle with a compression ratio of 20 between temperature limits of 25°C and 1400°C and an inlet pressure of 100 kPa. The power output is 500 kW.
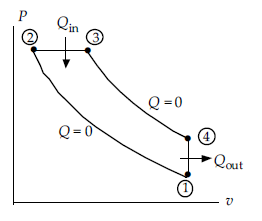
A) 1.69
B) 1.77
C) 1.84
D) 2.01
Which component may be used to adjust rear camber in many vehicles?
A) Strut rod B) Eccentric cam C) Eccentric bushing D) Tie rod end