How does the result compare with the corresponding equation for an ideal gas?
Z = 1 + B?P + C?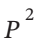 + D?
+ D?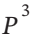 + . . .
+ . . .
Derive an equation for the work of mechanically reversible, isothermal compression of 1 mol of a gas from an initial pressure 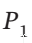 to a final pressure
to a final pressure 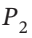 when the equation of state is the virial expansion truncated to:
when the equation of state is the virial expansion truncated to:
Z = 1 + B?P
We have, in general for a mechanically reversible process, dW = -PdV. If our equation of state is V = RT/P (1 + B’P) = RT /P + RTB’, then dV = -RT/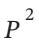 dP. So, dW = -P(-RT/
dP. So, dW = -P(-RT/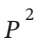 ) dP = RT/P dP. Integrating this from
) dP = RT/P dP. Integrating this from 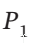 to
to 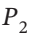 gives W = RT ln(
gives W = RT ln(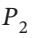 /
/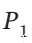 ),which is identical to the result for an ideal gas. The second term in this two-term form of the virial expansion does not affect the work of an isothermal expansion, because when we write the volume as an explicit function of pressure, the term containing the 2nd virial coefficient is independent of pressure.
),which is identical to the result for an ideal gas. The second term in this two-term form of the virial expansion does not affect the work of an isothermal expansion, because when we write the volume as an explicit function of pressure, the term containing the 2nd virial coefficient is independent of pressure.
You might also like to view...
If a contractor has a time extension that is justified, how is the amount of time determined?
What will be an ideal response?
A dimension of 50 millimeters should be shown as:
A. 50 B. 50.0 C. 50.00 D. 50.000
Voltage signals (waveforms) that do not go below zero are called __________
A) DC coupled signals B) Pulse trains C) DC signals D) AC signals
The temperature of the water returning to a chiller used for comfort cooling is typically about _____
a. 35°F b. 45°F c. 55°F d. 65°F