Energy in Nuclear Reactions: When a neutron collides with a uranium-235 nucleus it can induce a variety of fission reactions. One such reaction is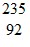 U +
U + 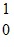 n ?
n ? 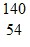 Xe +
Xe + 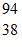 Sr + 2
Sr + 2 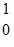 nThe following mass values are known:
nThe following mass values are known:
src="https://sciemce.com/media/4/ppg__cp1pbb0313192044__f440g1q11g6.jpg" style="vertical-align: -21.0px;" />Xe: 139.921620 u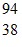 Sr: 93.915367 u
Sr: 93.915367 u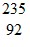 U: 235.043924 u
U: 235.043924 u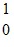 n: 1.008665 uHow much energy is released in this reaction? (1 u = 931.5 MeV/c2)
n: 1.008665 uHow much energy is released in this reaction? (1 u = 931.5 MeV/c2)
A. 185 MeV
B. 202 MeV
C. 32.6 MeV
D. 65.7 MeV
E. 98.6 MeV
Answer: A
Physics & Space Science
src="https://sciemce.com/media/4/ppg__cp1pbb0313192044__f440g1q11g6.jpg" style="vertical-align: -21.0px;" />Xe: 139.921620 u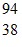 Sr: 93.915367 u
Sr: 93.915367 u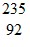 U: 235.043924 u
U: 235.043924 u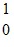 n: 1.008665 uHow much energy is released in this reaction? (1 u = 931.5 MeV/c2)
n: 1.008665 uHow much energy is released in this reaction? (1 u = 931.5 MeV/c2)
A. 185 MeV
B. 202 MeV
C. 32.6 MeV
D. 65.7 MeV
E. 98.6 MeV
Answer: A
You might also like to view...
A rock held above the ground has potential energy. As the rock falls, this potential energy is converted to kinetic energy. Finally, the rock hits the ground and stays there. What has happened to the energy?
A) The energy goes to producing sound and to heating the ground, rock, and surrounding air. B) The energy goes into the ground, and as a result, the orbit of the Earth about the Sun is slightly changed. C) The rock keeps the energy inside it in the form of mass-energy. D) It is transformed back into gravitational potential energy.
How much energy is released when 0.60 ?g of 3H have decayed to 3He?
The mass of 3He is 3.0160293 u, the mass of 3H is 3.0160492 u, 1 u = 931.5 MeV/c2, 1 eV = 1.60 × 10-19 J, and NA = 6.022 × 1023 molecules/mol.
Excimer laser radiation from KrF 248 nanometer (nm) broad beam can produce ozone from air.(76) Discuss whether the lofting of low-level satellites containing excimer lasers could be used to restore the ozone layer
What will be an ideal response?
Covalent bonding is caused by
A) the sharing of electrons between atoms. B) the transfer of electrons between atoms. C) unequal charge distributions around neutral molecules. D) atoms bonding to hydrogen molecules. E) atoms bonding to oxygen molecules.