Explain how electricity is produced in a fuel cell.
What will be an ideal response?
The way electricity is produced in fuel cells is always the same, more or less. A fuel comes in as a gas. It undergoes a chemical reaction at an electrode, usually with the help of a catalyst. The reaction produces an electron. The electron transfers into a wire and travels to another electrode to produce electricity. At the other electrode, another chemical reaction takes place to recombine the electron with an ion to balance the system out. While the general scheme of things is the same, the nuts-and-bolts of the processes differ, sometimes by quite a bit.
You might also like to view...
One kg of liquid water at 25°C, for which 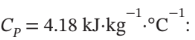
(a) Experiences a temperature increase of 1 K. What is 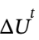 , in kJ?
, in kJ?
(b) Experiences a change in elevation ?z. The change in potential energy 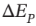 is the same as
is the same as 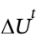 for part (a). What is ?z, in meters?
for part (a). What is ?z, in meters?
(c) Is accelerated from rest to final velocity u. The change in kinetic energy 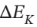 is the same as
is the same as 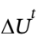 for part (a). What is u, in
for part (a). What is u, in 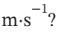
At 180°C, water has vf = 0.0011274 m3/kg and vg = 0.1941 m3/kg. A saturated mixture of water at this temperature has a quality of 0.25.
(a) Determine the specific volume of the water. (b) If the water has a mass of 1.5 kg, determine the total volume of the water. Given: vf = 0.0011274 m3/kg; vg = 0.1941 m3/kg; x = 0.25 What will be an ideal response?
Mosses contain vascular bundles
Indicate whether the statement is true or false
Labor and equipment pricing is based on _____
a. experience b. historical data c. plans and specifications d. contract clauses