A large ice cube is 30 cm on each side. If the length of each side is increased by a factor of three, the volume
A. increases by a factor of 1/9.
B. increases by a factor of 6.
C. increases by a factor of 9.
D. increases by a factor of 27.
D. increases by a factor of 27.
You might also like to view...
Fluid Flow: Ideal incompressible fluid flows through a 4.0-cm-diameter pipe at 1.0 m/s. There is a 2.0 cm diameter constriction in the line. What is the speed of the fluid in this constriction?
A. 0.25 m/s B. 0.50 m/s C. 2.0 m/s D. 4.0 m/s
Simple Pendulum: Two simple pendulums, A and B, are each 3.0 m long, and the period of pendulum A is T. Pendulum A is twice as heavy as pendulum B. What is the period of pendulum B?
A. T/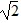
B. T
C. T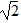
D. 2T
E. T/2
Two experimental runs are performed to determine the calorimetric properties of an alcohol which has a melting point of -10° C
In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C. The specific heat capacity of water is 4190 J/kg ? K. Assume no heat is exchanged with the styrofoam container and the surroundings. What is the specific heat capacity of the alcohol? A) 1700 J/kg ? K B) 1900 J/kg ? K C) 2100 J/kg ? K D) 2300 J/kg ? K E) 2500 J/kg ? K
The axis of a long hollow metallic cylinder (inner radius = 1.0 cm, outer radius = 2.0 cm) coincides with a long wire. The wire has a linear charge density of ?8.0 pC/m, and the cylinder has a net charge per unit length of ?4.0 pC/m. Determine the magnitude of the electric field 3.0 cm from the axis
a. 5.4 N/C b. 7.2 N/C c. 4.3 N/C d. 3.6 N/C e. 2.4 N/C